This article presents a complete SAT Chemistry study guide. I'll give you an overview of what's on the test, help you decide when to take it (or whether to take it at all), list sample questions and answers, and provide tips and resources that you can use in your studying. If you make use of the practice tools at your disposal and follow the general advice in this article, you'll be on your way to a great score!
UPDATE: SAT Subject Tests No Longer Offered
In January 2021, the College Board announced that, effective immediately, no further SAT Subject Tests will be offered in the United States. SAT Subject Tests ended internationally in June 2021. It is now no longer possible to take SAT Subject Tests, including the Subject Test in Chemistry.
Many students were understandably confused about why this announcement happened midyear and what this means for college applications going forward. Read more about the details of what the end of SAT Subject Tests means for you and your college apps here.
What's the Format of the Test, and When Is It Offered?
Here's a basic rundown of the structure and scoring of the Chemistry SAT Subject Test (also known as the Chemistry SAT II):- 60 minutes long
- 85 multiple-choice questions
- Scores range from 200 to 800
Note that subject tests still have quarter-point deductions for incorrect answers even though this policy has been abolished on the regular SAT. For every question you answer correctly you'll receive one point, no points will be awarded or deducted for questions left blank, and you'll lose 1/4 of a point for every incorrect answer. Calculator use is not permitted, but you'll have a periodic table to use for reference.
The Chemistry SAT Subject Test is offered in August, October, November, December, May, and June. In other words, you can take it on every regular SAT test date except the one in March.
Should You Take the SAT Chemistry Subject Test?
If you're reading this article, you're probably planning to apply to schools that require or recommend the submission of Subject Test scores. In most of these cases, it's best to take one Subject Test in the sciences and one in the humanities to show a range of skills.
Think about your goals, interests, and academic strengths before making a final decision on which science Subject Test you'll take.Chemistry is a great option for some students, but it might be less ideal for others.
Here are two reasons to choose Chemistry as one of your SAT Subject Tests:
Reason 1: You Just Took a Chemistry Class (and You Still Need a Science Subject Test)
The following are the College Board's recommendations for prerequisites before taking the Chemistry Subject Test:- One-year introductory college-preparatory course in chemistry
- One-year course in algebra
- Experience in the laboratory
If you just took a chemistry class (especially if it was AP), you probably won't need to do much studying for the Subject Test. The best time to take any Subject Test is in the spring when you're just finishing up a related class.
Reason 2: You're Super Interested in Chemistry
Do you think you might be a chemistry major in college? Do you have a knack for the subject? Taking the Subject Test is a good way to demonstrate your passion. If you have other achievements in high school that are related to chemistry, taking the Subject Test will help you present an even clearer picture of your goals and interests to colleges.
 A glimpse into your future. Or is it...your past? *cue Twilight Zone theme*
A glimpse into your future. Or is it...your past? *cue Twilight Zone theme*
What's on the Chemistry SAT II?
Here's a brief content overview of the Chemistry SAT Subject Test. I've listed the topics in descending order according to their prevalence on the test:
| Topic | Percentage of Test |
| Structure of matter (atomic structure, molecular structure, bonding) | 25% |
| States of matter (gases, liquids and solids, solutions) | 16% |
| Reaction types (acids and bases, oxidation-reduction, precipitation) | 14% |
| Stoichiometry (mole concept, chemical equations) | 14% |
| Descriptive chemistry (periodic trends, nomenclature, predicting products of reactions) | 12% |
| Lab practices (equipment and measurements, scientific method, data interpretation) | 8% |
| Thermochemistry (calorimetry, enthalpy and phase changes, entropy) | 6% |
| Equilibrium and reaction rates (equilibrium systems, rates of reactions) | 5% |
The College Board also breaks down questions by skill on this Subject Test:
|
Skill |
Percentage of Test |
| Application of knowledge | 45% |
| Synthesis of knowledge | 35% |
| Fundamental concepts and knowledge | 20% |
The most common questions, at 45 percent of the test, are application of knowledge questions. These questions ask you to apply your knowledge of chemistry to scenarios presented on the test.
Synthesis of knowledge questions, at 35 percent of the test, will ask you to draw conclusions based on data provided by the test as well as your background knowledge of chemistry.
Only twenty percent of questions test fundamental concepts and knowledge. These questions are focused on basic factual recall.
To break down these skills even further, questions will test your:
- Understanding of the major concepts of chemistry and ability to apply principles to solve specific problems
- Ability to organize and interpret results from observation and experimentation, and to draw conclusions or make inferences from experimental data, including data presented in graphs and/or tables
- Laboratory experience and familiarity with the metric system
- Ability to handle simple algebraic relationships and apply these to solving word problems
- Familiarity with the concepts of ratio, direct and inverse proportions, exponents, and scientific notations
Most of the test involves analysis and data interpretation. As I mentioned earlier, eighty percent of questions require either application or synthesis of knowledge, and only twenty percent are straightforward "what is this" type questions. In the next section, I'll provide examples of the various question formats you'll see on the test.

Question Formats on the Chemistry Subject Test
The College Board groups questions on the Chemistry SAT Subject Test into three different formats:
Format 1: Five-Choice Completion
These are what I would consider "normal" multiple choice questions. They're stand-alone questions that simply ask you to choose the correct answer out of five choices. In some cases, you'll get a list of three statements labeled with roman numerals and will be asked to decide which ones are true (if any). Here's an example of a question in that format:
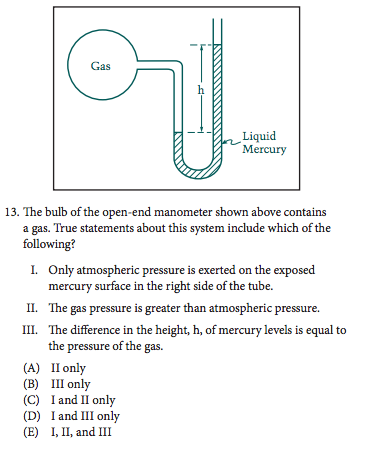
Before looking at the answer choices, we need to go through each of the statements and decide which ones are true based on the diagram:
- Statement I appears to be true because the exposed mercury surface is open to the air. There wouldn't be any pressure besides atmospheric pressure pushing down on that side.
- Statement II also appears to be true because the mercury level on the right side of the manometer is higher than the level on the left side.
- Statement III is not true because the pressure of the gas wouldn't be equal to h, it would be equal to the atmospheric pressure PLUS h. H is just the difference between the two pressures.
Now that we've decided only I and II are true, we can bubble in answer choice C!
Format 2: Classification
For these types of questions, you'll see one list of choices that applies to a group of several questions. Each choice can be used more than once or not at all. In other words, your answer to any individual question could also be the answer to other questions in a given group. Here's an example:

As you can see, questions that are formatted this way tend to be more straightforward. They're just identification questions. For this one, you need to understand how compounds are named and what their atomic symbols are.
Answers:
- A
- C
- A
In this case, one of the choices was used twice. This happens frequently, so don't freak out if you think a choice applies to more than one question in the group.
Format 3: Relationship Analysis
This is the weirdest type of question you'll see on the test. Each question is comprised of two statements that are connected to each other by the word "BECAUSE." The format is "This process or property occurs because of this fundamental chemical fact." Here's an example:
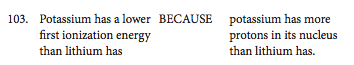
The first step is deciding whether each part of the statement is true or false. If both or either of the statements are false, you can ignore the Correct Explanation (CE) circle. If they're both true, you may have to fill in the special CE circle. However, that will not always be the case, so consider the logic of the full statement carefully even if both parts are true!
There will be a special section labeled "Chemistry" in the lower left-hand corner of your answer sheet where you can fill in your responses to these questions. It looks like this:
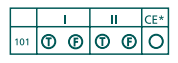
Your responses will look something like this:
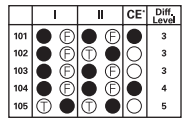
As you can see, for sample question 103, both statements are true, but the CE circle is left un-bubbled. Potassium has a lower first ionization energy than lithium, meaning it's easier for the one electron in its outer shell to get pulled away. Lithium's unpaired electron is closer to the nucleus, so it's held more tightly by the nucleus' gravitational pull. The first statement is definitely true.
Now let's look at the second statement in isolation: "potassium has more protons in its nucleus than lithium has." This is also true. Potassium has 19 protons, and lithium only has three.
Both statements are true on their own, but now we have to decide whether the second statement is a correct explanation of the first. In this case, it isn't!
The ionization energy doesn't increase based on the number of protons in the nucleus of the atom. It's a factor of how loosely held and how far away from the nucleus the outermost electrons of the atom are. Atoms that hold unpaired electrons that are farther away from the nucleus will have lower first ionization energies.
For lithium and potassium, both have unpaired electrons in their outermost orbitals, but potassium has a lower ionization energy because its electrons are farther away from the nucleus (4s orbital vs. 2s orbital). It's about proximity to the nucleus, not number of protons. The CE oval should stay blank!
Here are some electrons zooming around the nucleus of a particularly groovy atom.
Where to Find Chemistry Subject Test Practice Materials
Here I'll list a few different resources, both in print and online, that you can use to practice your skills for the SAT Subject Test in Chemistry. You should always return to official College Board questions (which you can find on the College Board website as the first listing under Online Resources) in your studying to verify that you're up to speed with the format of the test, but unofficial practice is still helpful for learning the content.
Review Books
If you want to add structure to your studying, you might consider buying a review book or a book of practice questions. A review book containing content that's specifically tailored to the Chemistry SAT II can improve the efficiency of your studying. Here are some of the most popular ones:
- Barron's SAT Subject Test Chemistry 14th Edition
- Cracking the SAT Chemistry Subject Test (Princeton Review)
- Sterling Test Prep SAT Chemistry Practice Questions
- Official SAT Subject Test in Chemistry Study Guide
Online Resources
College Board Website
You can practice questions in all the formats you'll see on the test here. There are only 13 questions, but they can help you get a feel for the test. You should also check out this document that has all the information you need for the test (and other subject tests if you're interested) along with more sample questions.
CrackSAT
There are tons of different quizzes here on various topics in chemistry. You won't find any of the Relationship Analysis true-false questions, but this is still overall a pretty good resource for regular multiple choice questions.
Albert.IO
This site has practice questions for all topics you might come across on the exam. Questions are also divided by difficulty level. Once you get all the hard questions right, you'll know that you've truly mastered the material.
SparkNotes
SparkNotes has practice quizzes on every topic with detailed answer explanations. These quizzes aren't automatically scored like some others on this list, but the way it goes over the reasoning for each choice in detail makes it a worthwhile resource.
ReasonPrep YouTube Videos
These videos walk you through the answers to sample questions found on official practice tests and in the official review book.
5 SAT Chemistry Study Tips
This section lists five study strategies that are critical for this test. You should have these tips in the back of your mind at all times as you review the material!
Tip 1: Identify Your Weaknesses
The Chemistry SAT II may test some information that your teacher didn't cover in class. Review all the content areas listed above to make sure you have a handle on everything. It's a smart idea to take a diagnostic test before you start studying to form a clearer picture of where your knowledge is lacking. I'd recommend using one that comes directly from the College Board so that the content and format of the test is represented accurately.
Tip 2: Replicate Test Conditions
When you answer practice questions and take practice tests, adhere to the same standards as the real test environment. Don't use a calculator, and have a periodic table on hand for reference. If you're taking a full practice test, you should also time yourself. Sticking to appropriate test conditions is the only way to reliably estimate your score level!
Tip 3: Plan Your Time Wisely
Your studying timeline should be informed by how recently you learned the material in class and how well you perform on an initial diagnostic test. If you find that you need to improve by 150 points or more, plan to spend some extra time reviewing the material. I'd estimate about 20 hours of study time to get yourself up to speed - if you start two months in advance, that's just two hours a week. Not so bad!
If you're already within 50 points or so of where you want to be, a quicker review should suffice. You could probably spend less than ten hours going over the material. Make sure you get to a point where you feel very confident with the question formats in case nervousness interferes with your performance (which brings me to the next tip).
Tip 4: Practice Relationship Analysis Questions
Make sure you understand how these questions work prior to the test. They take a little bit of getting used to if you've never encountered them before. Try to use some practice resources that include these types of questions in addition to five-choice completion and classification questions. It's especially important to practice interpreting the statements together and on their own and keeping the two processes separate. That CE circle is a little bit tricky!
Tip 5: Don't Go Overboard
You should only study concepts that you know will be tested. There's no need to memorize a year's worth of material for an hour-long multiple-choice test. I'd recommend dividing your time equally between reviewing topics in your notes and doing practice questions that replicate the format of the subject test.
The biggest challenge for you may be time rather than background knowledge, especially if you're fresh out of a chemistry class. You shouldn't focus too much on memorizing all your chemistry notes and doing involved practice problems. Remember, you can't even use a calculator on this test, so you won't have to solve any elaborate math problems.
 "What's that weird squiggly?" and "Why are there little numbers next to the letters?" are both questions you should be able to answer before taking this Subject Test.
"What's that weird squiggly?" and "Why are there little numbers next to the letters?" are both questions you should be able to answer before taking this Subject Test.
4 Test-Taking Tips for SAT Chemistry
This section lists four additional tips that you can implement as you take practice tests (and, ultimately, when you sit down for the real thing).
Tip 1: Stay Calm
Since you can't use a calculator on the test, many of these questions involve combining common sense with basic chemistry knowledge. If you come across a question about a diagram or experimental scenario that you've never seen before, don't freak out. Just read the question carefully. In almost all of these cases, you'll find that your existing knowledge is enough to guide you to the correct answer. Even if you don't fully understand what's going on in the experiment, if you have a solid understanding of chemistry, you can make deductions to arrive at the solution.
Tip 2: Do the Easiest Classification Questions First
When you get to a group of classification questions, answer the ones that seem obvious first so you're using your time efficiently. Don't feel like you have to complete them in order. If you get the easy ones out of the way in ten seconds, you'll have more time to think about the questions in the group that are more challenging for you.
Tip 3: Read Relationship Analysis Questions Methodically
Relationship Analysis questions are different from most questions on SAT Subject Tests, so they can be confusing. Read carefully, and decide whether each stand-alone statement is true before thinking about whether the second statement is a correct explanation of the first. You'll save yourself some time if you find that one of the statements is false (and makes the CE bubble irrelevant)!
Tip 4: Don't Guess Randomly
As I mentioned in the first section of this article, the guessing penalty still exists on SAT Subject Tests. This means that you need to avoid guessing if you have no clue which answer choice is correct. Guessing is only advisable if you can narrow down your choices to four or fewer possibilities. Otherwise, you're better off leaving the question blank.
 Come back here, metaphor for the correct answer choice!
Come back here, metaphor for the correct answer choice!
Conclusion
The SAT Chemistry Subject Test is an hour-long multiple-choice exam that contains 85 questions and is scored out of 800. It's offered on all the regular SAT test dates except March.
Topics on the test include:
- Structure of matter
- States of matter
- Reaction types
- Stoichiometry
- Descriptive chemistry
- Lab practices
- Thermochemistry
- Equilibrium and reaction rates
Most questions will test your ability to analyze different experimental scenarios and draw conclusions. The questions come in three formats:
- Five-choice completion
- Classification
- Relationship analysis
When you study for the test, five tips to keep in mind are:
- Identify your weaknesses
- Replicate real testing conditions
- Plan out your studying wisely
- Practice lots of relationship analysis questions
- Don't over-study
When you take the test (or a full practice test), you should be sure to:
- Stay calm
- Do easier classification questions first
- Read carefully on relationship analysis questions
- Avoid guessing unless you're down to just two choices
Use the online resources and review books listed in this article to brush up on your skills. You should have no problem acing this test if you know what to expect!
What's Next?
Get started on your chemistry reviewing with our guide to balancing chemical equations. Alternatively, apply chemistry to your life by learning how to make three different types of slime or how to use muriatic acid for household cleaning.
Are you thinking you might take the Biology Subject Test instead of Chemistry? Do you plan on taking both? If so, check out my ultimate study guide for SAT Biology E/M!
You might be wondering whether AP tests or SAT Subject Tests carry more weight in the admissions process. Learn more about the differences between AP tests and SAT Subject Tests, and find out how much each type matters for you.
If you're still on the fence about whether or not to take the Chemistry Subject Test, read our expert guide to help you decide which Subject Tests are your best bets based on your college goals.
These recommendations are based solely on our knowledge and experience. If you purchase an item through one of our links, PrepScholar may receive a commission.
Have friends who also need help with test prep? Share this article!

Samantha is a blog content writer for PrepScholar. Her goal is to help students adopt a less stressful view of standardized testing and other academic challenges through her articles. Samantha is also passionate about art and graduated with honors from Dartmouth College as a Studio Art major in 2014. In high school, she earned a 2400 on the SAT, 5's on all seven of her AP tests, and was named a National Merit Scholar.

































 Holly R.
Holly R.