One of the first science experiments I remember was adding salt to a cup of water and waiting eagerly for it to dissolve. Though I was excited to watch the salt seem to “disappear” I definitely didn’t understand the intricacies of solubility. Luckily, solubility follows a list of rules that helps us determine how soluble a substance is, like how likely that salt is to dissolve into that water (sneak peek- it’s very likely). We’re going to go over what solubility is, how it works, and the complete list of solubility rules to help you determine the solubility of substances.
What Is Solubility?
Solubility is a substance's ability to be dissolved. The substance that is dissolved is called a solute, and the substance it is dissolving in is called a solvent. The resulting substance is called a solution. Generally, the solute is a solid and the solvent is a liquid, such as our salt in water example above. However, solutes can be in any state: gas, liquid, or solid. For example, a carbonated beverage is a solution where the solute is a gas and the solvent is a liquid.
A solute is considered insoluble when they are unable to dissolve at a ratio greater than 10000:1. While many compounds are partially or mostly insoluble, there is no substance that is completely insoluble in water, meaning that it can't dissolve at all. You will see in the solubility rules that many compounds that are labeled as insoluble have exceptions, such as carbonates. This is partly why it's important to follow the solubility rules closely.
When you are working on chemical equations or building a hypothesis, solubility rules are helpful in predicting the end states of the substances involved. You will be able to accurately predict what combinations will lead to what results.
The solubility rules are only for ionic solids' ability to dissolve in water. While we can calculate the solubility by measuring each substance and following an equation, the solubility rules allow us to determine the solubility of a substance before you attempt to create it.
Solubility Rules
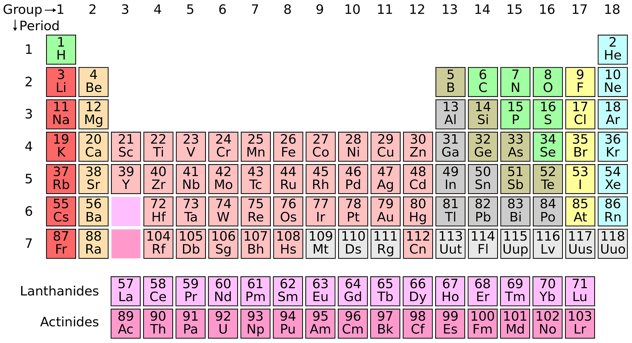
It is very important that the rules on this list are followed in order, because if a rule seems to contradict another rule, the rule that comes first is the one that you follow. Substances on this list are given by their elemental names. Referencing the periodic table below will help you work through the elemental names and groups.
-
Salts containing Group I elements (Li+, Na+, K+, Cs+, Rb+) are soluble . There are few exceptions to this rule. Salts containing the ammonium ion (NH4+) are also soluble.
-
Salts containing nitrate ion (NO3-) are generally soluble.
-
Salts containing Cl -, Br -, or I - are generally soluble. Important exceptions to this rule are halide salts of Ag+, Pb2+, and (Hg2)2+. Thus, AgCl, PbBr2, and Hg2Cl2 are insoluble.
-
Most silver salts are insoluble. AgNO3 and Ag(C2H3O2) are common soluble salts of silver; virtually all others are insoluble.
-
Most sulfate salts are soluble. Important exceptions to this rule include CaSO4, BaSO4, PbSO4, Ag2SO4 and SrSO4 .
-
Most hydroxide salts are only slightly soluble. Hydroxide salts of Group I elements are soluble. Hydroxide salts of Group II elements (Ca, Sr, and Ba) are slightly soluble. Hydroxide salts of transition metals and Al3+ are insoluble. Thus, Fe(OH)3, Al(OH)3, Co(OH)2 are not soluble.
-
Most sulfides of transition metals are highly insoluble, including CdS, FeS, ZnS, and Ag2S. Arsenic, antimony, bismuth, and lead sulfides are also insoluble.
-
Carbonates are frequently insoluble. Group II carbonates (CaCO3, SrCO3, and BaCO3) are insoluble, as are FeCO3 and PbCO3.
-
Chromates are frequently insoluble. Examples include PbCrO4 and BaCrO4.
-
Phosphates such as Ca3(PO4)2 and Ag3PO4 are frequently insoluble.
-
Fluorides such as BaF2, MgF2, and PbF2 are frequently insoluble.
Sample Questions
1. Select the compounds that are always soluble in water
a. BaSO4
b. HG2 I2
c. Na OH
d. Na2 SO3
e. Ag ClO3
f. Cr Cl3
g. Fe PO4
2. Label each of the following as soluble or insoluble
a. Li OH
b. Hg SO4
c. Pb Br2
d. Rb2 S
e. Ni I2
f. H3 AsO4
g. Ni Cro4
3. Which (if any) silver is soluble: Silver chloride AgCl, silver phosphate, Ag3 PO4, or silver fluoride, AgF?
Answers
1. Select the compounds that are always soluble in water (bolded are correct)
a. BaSO4 (see rule 5)
b. HG2I2 (see rule 3)
c. Na OH (see rule 1)
d. Na2 SO3 (see rule 1)
e. Ag ClO3 (see rule 3)
f. Cr Cl3 (see rule 3)
g. Fe PO4 (see rule 6)
Note: Letter e is an example of using the order of the rules to determine solubility. Rule 4 says that silvers (Ag) are frequently insoluble, but rule 3 says that chlorates (Cl) are soluble. Since Ag ClO3 is a silver chlorate, and rule 3 comes before rule 4, it supersedes it. This compound is soluble.
2. Label each of the following as soluble or insoluble
a. Li OH soluble- rule 1
b. Fe (OH)2 insoluble- rule 7
c. Pb Br2 insoluble – rule 2
d. Rb2 SO3 soluble- rule 1
e. Ni I2 soluble – rule 3
f. H3 AsO4 insoluble- rule 10
g. Ni CRo4 insoluble- rule 8
3. Which (if any) silver is soluble: Silver chloride AgCl, silver phosphate, Ag3 PO4, or silver fluoride, AgF?
None of the above silver is soluble. In rule #4, it states that silver salts (Ag) are
insoluble, with silver nitrate, AgNO3, as one exception.

How Solubility Works
As we see from our solubility rules, some substances are very soluble, while some are insoluble or have low solubility. Let's take a look at how solubility works to better understand the solubility rules.
Factors That Affect Solubility
Whether or not a substance is soluble, and to what degree, depends on a variety of factors. Solutes typically will dissolve best in solvents that have the most molecular similarities. Polarity is a major factor in a substance's solubility. Molecules where one end is negatively charged and the other is positively charged are considered “polar,” meaning that they have electrical poles. If a molecule does not have this ionic makeup, it is considered nonpolar.
Generally, solutes are soluble in solvents that are most similar to them molecularly. Polar solutes will dissolve better in polar solvents, and non-polar solutes will dissolve better in non-polar solvents. For example, sugar is a polar solute, and absorbs very well in water. However, sugar would have a low solubility in a nonpolar liquid like vegetable oil. In general, solutes will also be more soluble if the molecules in the solute are smaller than the ones in the solvent.
Other factors that affect solubility are pressure and temperature. In some solvents, when heated the molecules vibrate faster and are able to break apart the solute. Pressure is mainly a factor when a gas substance is involved, and has little to no effect on liquid substances.
The rate of solution refers to how quickly a substance dissolves, and is separate from solubility. Solubility depends entirely on the physical and chemical properties of the solute and solvent, and isn’t affected by the rate of solution. Rate should not be factored into the solubility of a substance.This can often be confusing when first learning about solubility, since in a visual example, watching something dissolve quickly can feel like an affirmation of its ability to dissolve. However, the process of solubility is unique, and the rate at which it dissolves is not factored into the equation.
Predicting Outcomes
When a solute is mixed with a solvent, there are three possible outcomes: If the solution has less solute than the maximum amount it is able to dissolve (the solubility), it is a dilute solution. If the amount of solute is exactly the same as the solubility it is saturated. If there is more solute than is able to be dissolved, the excess separates from the solution and forms a precipitate.
A solution is considered saturated when adding additional solute does not increase the concentration of the solution. Additionally, a solution is miscible when it can be mixed together at any ratio- this mainly applies to liquids, like ethanol, C2H5OH, and water, H2O.
Knowing and following the solubility rules is the best way to predict the outcome of any given solution. If we know that a substance is insoluble, it is likely that it would have excess solute, thus forming a precipitate. However, compounds that we know to be highly soluble, like salt, are likely to form solutions at various ratios; in this case, we will be able to determine how much solute and solvent is needed to form each solution, and if it's possible to form one at all.
Thinking about the salt in water experiment now, it’s clear that the salt- also known as NaCl or sodium chloride, would be highly soluble according to our solubility rules. Sodium chloride contains Na, which is almost always soluble according to rule 1, and Cl, which is usually soluble according to rule 3. Though I can tell this just by glancing at the rules, nothing takes away from the magic of watching chemical compounds break down and dissolve right before your eyes. Remember to keep your periodic tables handy, and pay close attention to the solubility rules in your next experiment.
What's Next?
Preparing for the AP Chemistry test? Study with our articles on every AP Chemistry practice test available and the ultimate AP Chem study guide. Taking IB instead? Start with our study notes for IB Chemistry.
Looking for more chemistry help? We walk you through the solubility constant (Ksp) and how to solve for it, explain how to balance chemical equations, and go over examples of physical vs chemical change here.
If you need more non-chemistry science guides, be sure to check out these guides about finding the density of water, defining commensalism, and how to calculate acceleration.